How can we reliably identify "smoldering" disease in multiple sclerosis using MRI?
What are the underlying mechanisms of this progressive process?

Paramagnetic (Iron) Rim Lesions
Multiple Sclerosis (MS) is characterized by the accumulation of areas of inflammatory injury ("lesions") to the brain and spinal cord. In the aftermath of a new lesion formation, the brain and immune system attempt to heal. In some cases — for reasons yet unknown — a lesion will fail to repair and instead develop ongoing, low-grade inflammation. In these types of "chronic active lesions", cells at the border (rim) of the lesion will often sequester iron, a paramagnetic molecule that can be readily visualized using special MRI sequences. These iron-laden cells tend to be highly inflammatory. They can be visualized as a dark border around a typical MS lesion. Many questions surround the role of these lesions in MS: what is the natural history of these lesions? Do they predict certain clinical outcomes? Can they be treated by any of our current therapies? We have recently shown that these lesions are highly specific for a diagnosis of MS. We collaborate closely with Daniel Reich at the NIH Translational Neuroradiology Section as well as with Sathish Dundamadappa at UMass Radiology and Alex Rauscher at University British Columbia.
Quantitative Volumetry
Measuring the volume and appearance of brain and spinal cord structures can yield important insights about the health of the underlying tissue. Abnormal shrinkage of certain brain areas early in the course of multiple sclerosis (MS) can herald clinical decline. For example, the thalamus is one of the earliest structures to shrink in MS and we collaborate with the Saranathan Lab in assessing thalamic subnuclear segmentation. We employ a wide variety of tools to accurately quantify brain structures. Among many, these can include Freesurfer, FSL, SPM, C3D, ITK-SNAP, 3D Slicer, and ANTs. We are now implementing newer deep learning segmentation tools that can be trained on relatively small datasets as well. The Principal Investigator (PI) runs a longitudinal, observational study of MS (the OPTIMUM study) to study real-world information from persons with MS, a resource that allows new insights into the disease by combining MRI with clinical data informatics.

Fluid Biomarker Development
Our close colleague and collaborator Carolina Ionete, MD, PhD, runs the UMass Chan MS biorepository. We often combine forces to leverage imaging assessments together with fluid (cerebrospinal fluid, urine, and blood) analysis to gain deep biological insights into proteomic and cellular immunology underlying multiple sclerosis and its imaging features. Recent techniques have included attomolar-sensitivity proteomics and multiplexed ELISAs investigating associations of MS with reactive oxidative stress.
Neural Immune Interfaces
The immune system interfaces with the brain at many places. These meeting points are important areas in which the immune cells can be influenced or programmed. We are interested in examining these areas using MRI, to potentially identify biomarkers for areas propagating smoldering infammatory disease. These areas may include the meningeal compartments, circumventricular tissues, the cervical lymph nodes or even the skull bone marrow.
Credit: NIAID
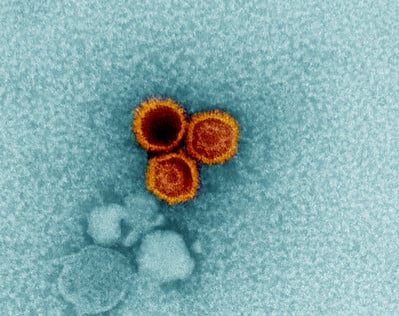
Epstein-Barr Virus
The Epstein-Barr Virus (EBV) has been demonstrably linked to multiple sclerosis as a necessary but highly insufficient factor. Essentially 100% of MS patients show evidence of a prior infection (although >90% of the health adult population does as well). What is EBV doing to the immune system to cause this predisposition toward MS? We collaborate with the Selin Lab to assess EBV T-cell (dys)regulation and the Chan Lab to explore how EBV may contribute to an autoimmune antibody response.
Neural top-down regulation
The brain can influence the immune system throughout the body via the autonomic nervous system. Situations of high stress and adversity have been linked to an elevated risk of multiple sclerosis (MS) flare. Conversely, one randomized controlled trial showed that stress reduction reduced the risk of new lesion formation. We are interested in these connections and exploring the biology underlying how conditions of adversity can shift the immune response toward a more global pro-inflammatory response. We collaborate with the mindfulness program at UMass and the UCLA Cousin's Center for Psychoneuroimmunology. For an example of this work, see this publication.